Why are makers of Alzheimer’s ‘wonder drug’ so silent over its side effects?

It was whereas collaborating in a medical trial for a brand new dementia ‘wonder drug’ that Genevieve Lane, 79, suffered a deadly mind seizure.
Her demise, reported by The Mail on Sunday final week, got here amid nice hope for lecanemab, which specialists had hailed as ‘the beginning of the end’ for Alzheimer’s – the degenerative illness that blights the lives of 1,000,000 Britons.
But it additionally emerged that Genevieve, from Florida, was only one of three individuals who have died from issues which can probably be linked to lecanemab, and has led to some specialists calling for a pause to its rollout.
The drug is at the moment being assessed by the NHS spending watchdog, the National Institute for Health and Care Excellence (NICE), which can quickly determine if it will likely be provided to tens of 1000’s of Britons.
Now issues about lecanemab are rising deeper. Today we will reveal additional startling revelations that solid extra doubt on the integrity of the information used to show the drug’s security.
Genevieve Lane, a retired automotive rent supervisor was first identified with dementia two years earlier in 2020, after she observed small lapses in her reminiscence

Ms Lane, pictured together with her daughters, died in September 2022 after her third dose of Alzheimer’s marvel drug lecanemab
And specialists are now telling The Mail on Sunday they imagine rolling out lecanemab within the UK might put lives in danger.
The points raised embrace:
lThat the severity of attainable harmful side results, resembling mind shrinkage, could have been underestimated;
lAlzheimer’s docs have accused the drug producer, Eisai, of withholding essential info, together with particulars of three sufferers who died after taking the drugs;
lClinicians have referred to as for the complete security information to be obtainable earlier than US regulators meet subsequent month to determine whether or not to approve lecanemab, though Eisai is below no authorized obligation to do that;
lInsiders have raised issues that Eisai did not correctly examine Genevieve’s demise, that means the complete extent of any side impact are not identified;
lThe agency reportedly waited two months earlier than informing US well being authorities of the demise – usually, trial deaths should be reported inside per week.
Such is the fear over lecanemab that some specialists are calling for an ‘urgent re-evaluation’ of the information collected by Eisai which was used to show its security.
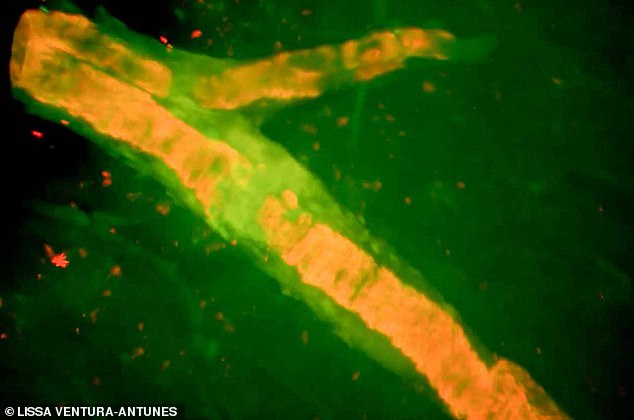
Ms Lane suffered a large bleed on the mind, proven right here on this scan

Research has proven that one in six sufferers handled with the ‘marvel drug’ undergo from mind bleeds whereas not less than three of the folks concerned within the scientific trial died
The drug first attracted public consideration in September when Eisai revealed the outcomes of a significant trial involving 1,800 contributors and carried out over 18 months at 240 websites the world over, together with the US, UK and Australia. It was discovered to gradual cognitive decline by a 3rd and was the primary therapy confirmed to delay development of the illness
‘The drug company has said lecanemab is safe, but these deaths could suggest otherwise,’ says Robert Howard, professor of outdated age psychiatry at University College London’s Institute of Mental Health.
‘There is a credible risk that, if rolled out on the NHS, more lives could be put in danger. Which is why there is a moral imperative to release the full data.’
Other mind specialists agree. ‘The handling of the deaths raises doubts as to whether serious lecanemab side effects are being spotted and properly reported,’ says Dr Matthew Schrag, a neurologist at Vanderbilt University in Nashville, Tennessee, who carried out Genevieve’s post-mortem.
‘There are question marks over whether the published safety data is representative of the facts. It is crucial that Alzheimer’s docs see this info earlier than this drug is authorized.’
The drug first attracted public consideration in September when Eisai revealed the outcomes of a significant trial involving 1,800 contributors and carried out over 18 months at 240 websites the world over, together with the US, UK and Australia. It was discovered to gradual cognitive decline by a 3rd and was the primary therapy confirmed to delay development of the illness.
Professor John Hardy, a dementia researcher at University College London, declared the findings the ‘beginning of the end’ for Alzheimer’s and stated he hoped it could be obtainable on the NHS inside a 12 months.
Lecanemab, given each two weeks as a drip by way of a vein within the arm till it stops working, clears a protein often called amyloid which builds up within the brains of Alzheimer’s sufferers. Amyloid clumps are thought to trigger the progressive mind harm seen in dementia.
But preliminary trials revealed that in some instances lecanemab could cause undesirable issues within the mind – together with swelling, bleeding and irritation, a situation identified medically as amyloid-related imaging abnormalities, or ARIA. About one in ten contributors within the trial developed mind swelling and one in six suffered mind bleeds, in line with Eisai information.
Trial contributors had been monitored with MRI mind scans each three months, and those that developed ARIA had been taken off the drug. The majority had no signs.
Eisai stated lecanemab was not associated to an elevated danger of demise because the fatality fee was much like that of the group within the trial who got a placebo.
‘No deaths were considered by the investigators to be related to lecanemab or occurred with ARIA’, stated the research’s authors, from Yale University.
But post-mortem investigators and an investigator on the trial have now recognized three instances, together with that of Genevieve Lane, the place lecanemab sufferers died of sudden signs doubtless related to the drug. One case occurred throughout the trial, the opposite two after it had been accomplished.
In two instances, the deaths had been stated to be associated to an interplay between lecanemab and blood thinners – medicines which scale back the chance of strokes and coronary heart assaults however can enhance bleeding danger.
Genevieve was not on blood thinners and was comparatively wholesome. But shortly after her third dose, she suffered a large seizure. Five days later, she died. An post-mortem discovered lecanemab was the most probably trigger of demise. In all three instances, Eisai has both denied the demise was linked to the drug or declined to remark.
Speaking to the MoS, Genevieve’s household declare the trial docs – who labored for an impartial scientific trial firm employed by Eisai – missed very important warning indicators, together with worrying signs, that will have prevented her demise.
‘We believe Mum told her doctor about headaches when she returned for her second dose. But he still proceeded to give her the treatment and did not suggest carrying out any scans,’ says Genevieve’s youthful daughter, Charlotte Lane, 56. ‘It’s surprising to assume that, on the trial of an experimental drug, it looks as if nobody was looking for worrying signs.’
The household imagine that the trial docs did not correctly examine their mom’s demise.
When her youngsters wished to know extra about what had led to her demise, ‘we were surprised the trial company didn’t present any curiosity in finishing up an post-mortem,’ says Charlotte. ‘It didn’t look like they wished to analyze something extra.’
Genevieve’s daughters finally contacted researchers at Vanderbilt University, who agreed to hold out an post-mortem.
Eisai stated it couldn’t touch upon particular person contributors. ‘The wellbeing of patients enrolled in our studies is always Eisai’s high precedence,’ stated a spokesman. ‘Eisai has established rigorous safety monitoring process. This includes an independent data safety monitoring committee of external experts. Eisai promptly communicated important safety information to regulatory agencies, sites, investigators and subjects.’
But some specialists have questioned this. One neurologist aware of Genevieve’s case raised issues her demise could not have been reported to the US medicines watchdog, the Food and Drug Administration (FDA), till December 2022 – almost two months after Eisai introduced lecanemab was protected and efficient.

The different individuals who died throughout the trial had been taking blood thinners – medicines that assist scale back the chance of strokes and coronary heart assaults however can enhance the chance of bleeding. Until now, specialists have believed it was the mix of lecanemab and blood thinners that raised the chance of life-threatening side results
Eisai declined to remark. An FDA spokesman additionally declined to say when the data was obtained, citing ‘patient privacy’.
Researchers usually carefully monitor sufferers after research finish, to maintain be aware of potential long-term side results, often called an extension trial. Eisai will acquire information on their lecanemab trial contributors till 2027.
Seven months on from the discharge of the preliminary trial outcomes, Eisai has to this point declined calls to publish any follow-up information, which would come with two of the deaths.
Speaking at a significant neurology convention final week in Boston, US, lead investigator of the lecanemab trial, Professor Christopher van Dyck, wouldn’t reply questions or present details about its extension trial outcomes. Eisai additionally declined the MoS’s request to see the information.
Experts say it’s essential this info is made public as quickly as attainable.
Meanwhile, latest proof has sparked additional issues about lecanemab. Last month, Australian researchers revealed a research displaying sufferers taking anti-amyloid medicine, together with lecanemab, skilled accelerated mind shrinkage. This is commonly an indicator that cognitive decline is worsening. Patients taking the medicine noticed their brains shrink 40 per cent quicker than those that didn’t have any therapy.
‘We currently don’t know which sufferers are most in danger of these types of side results as a result of we haven’t seen info that tells us about traits that may have made them extra susceptible,’ says Professor Scott Ayton, dementia knowledgeable on the Florey Institute of Neuroscience and Mental Health in Melbourne, Australia.
But one group, who carry a gene referred to as APOE4 which has lengthy been linked to dementia, could possibly be six instances extra prone to see mind swelling after taking lecanemab and thrice as prone to expertise mind bleeds, in line with a research within the medical journal Jama Neurology final month.
Researchers analysed obtainable Eisai information, which confirmed genetic traits of sufferers who suffered extreme side results.
Importantly, research estimate as much as 65 per cent of Alzheimer’s sufferers carry the APOE4 gene and could also be in danger.
‘We need to know whether patients with these genes who suffered bad side effects also had underlying conditions, so we know exactly who should not be taking lecanemab,’ says Dr Madhav Thambisetty, a neurologist on the US National Institute of Aging. ‘Researchers have asked for this information time and time again – and Eisai has refused.’
Genevieve’s household share these frustrations. They are but to obtain her full medical information from Eisai, although they had been requested in January. Last month the agency lastly agreed to ship the paperwork, however they haven’t but arrived.
‘We felt ignored for months after Mum died,’ says Charlotte. ‘It’s solely now we’ve began speaking publicly about her demise that they’ve agreed to something.
‘We deserve to know what happened. There are missing pieces of the puzzle. We want someone to take responsibility.’
- Some names have been modified for privateness causes.